UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT PURSUANT TO
SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported): August 13, 2018

AMICUS THERAPEUTICS, INC.
(Exact Name of Registrant as Specified in Its Charter)
Delaware
(State or Other Jurisdiction of
Incorporation)
001-33497 (Commission File Number) | 71-0869350 (IRS Employer Identification No.) |
1 Cedar Brook Drive, Cranbury, NJ (Address of Principal Executive Offices) | 08512 (Zip Code) |
Registrant’s telephone number, including area code: (609) 662-2000
(Former Name or Former Address, if Changed Since Last Report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
o | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
o | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
o | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
o | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company o
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Item 8.01 Other Events
On August 13, 2018, Amicus Therapeutics, Inc. will be holding a conference call to discuss the FDA's approval of Galafold™ (migalastat) for the treatment of certain adult patients with Fabry disease. A copy of the conference call presentation materials is attached hereto as Exhibit 99.1.
Item 9.01 | Financial Statements and Exhibits |
(d) Exhibits:
Exhibit No. | Description |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
AMICUS THERAPEUTICS, INC. | |
Date: August 13, 2018 | By: /s/ Ellen S. Rosenberg |
Name: Ellen S. Rosenberg | |
Title: General Counsel and Corporate Secretary | |

Galafold™ (Migalastat) U.S. Approval Call August 13, 2018

Introduction 2 Safe Harbor This presentation contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 relating to approval and commercialization plans for Galafold in the United States. The inclusion of forward-looking statements should not be regarded as a representation by us that any of our plans will be achieved. Any or all of the forward-looking statements in this press release may turn out to be wrong and can be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties. For example, actual results may differ materially from those set forth in this release due to the risks and uncertainties inherent in our business, including, without limitation: the potential that we may not be successful in commercializing Galafold in the United States, the potential that public and commercial payors will not reimburse Galafold, the potential that we may not be able to manufacture or supply sufficient commercial products; and the potential that we will need additional funding to complete all of our commercialization and manufacturing activities. In addition, all forward- looking statements are subject to other risks detailed in our Annual Report on Form 10-K for the year ended December 31, 2017 as well as our Quarterly Report on Form 10-Q for the quarter ended June 30, 2018 filed August 7, 2018 with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward- looking statements are qualified in their entirety by this cautionary statement, and we undertake no obligation to revise or update this news release to reflect events or circumstances after the date hereof.

Introduction 3 Our Passion for Making a Difference Unites Us

Galafold: Precision Medicine for Fabry Disease 4 Galafold (Migalastat) Launch Progress (8/10/18) Patients have Reimbursed Access in 20 Countries Switzerland E.U. Canada Japan United States Israel Taiwan S. Korea = approved Australia = pending approvals

Galafold: Precision Medicine for Fabry Disease 5 U.S. Label Highlights Galafold Approved under Subpart H Accelerated Approval Pathway . Galafold is indicated for the treatment of adults with a confirmed diagnosis of Fabry disease and an amenable galactosidase alpha gene (GLA) variant based on in vitro assay data . This indication is approved under Accelerated Approval based on reduction in kidney interstitial capillary cell globotriaosylceramide (KIC GL-3) substrate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. . Approved for 348 amenable variants . The most common side effects: headache, nasopharyngitis, urinary tract infection, nausea and pyrexia

Galafold: Precision Medicine for Fabry Disease 6 U.S. Demographics for Galafold Launch Underway NDA Approved (Priority Review) Ahead of August 13, 2018 PDUFA Full launch team hired and trained Appropriate stakeholder engagement ongoing >3,000 diagnosed (~1,500 treated) (US Estimates) ~35-50% amenable (Global Estimates) Orphan Drug and Fast Track designations

Galafold: Precision Medicine for Fabry Disease 7 Leveraging Our Operations Excellence Full team hired, Brings great experience Eager to introduce Team trained and in place Galafold to the U.S. across the U.S and passion Patients & Creating awareness of oral treatment option and label Physicians US Launch Focus Compelling Commitment to Ensuring broad market Access value patient access and access aligned with proposition support services indication Clear focus at Efficient outreach Strong education Execution launch on priority to key Fabry efforts on importance patient segments disease centers of genotype
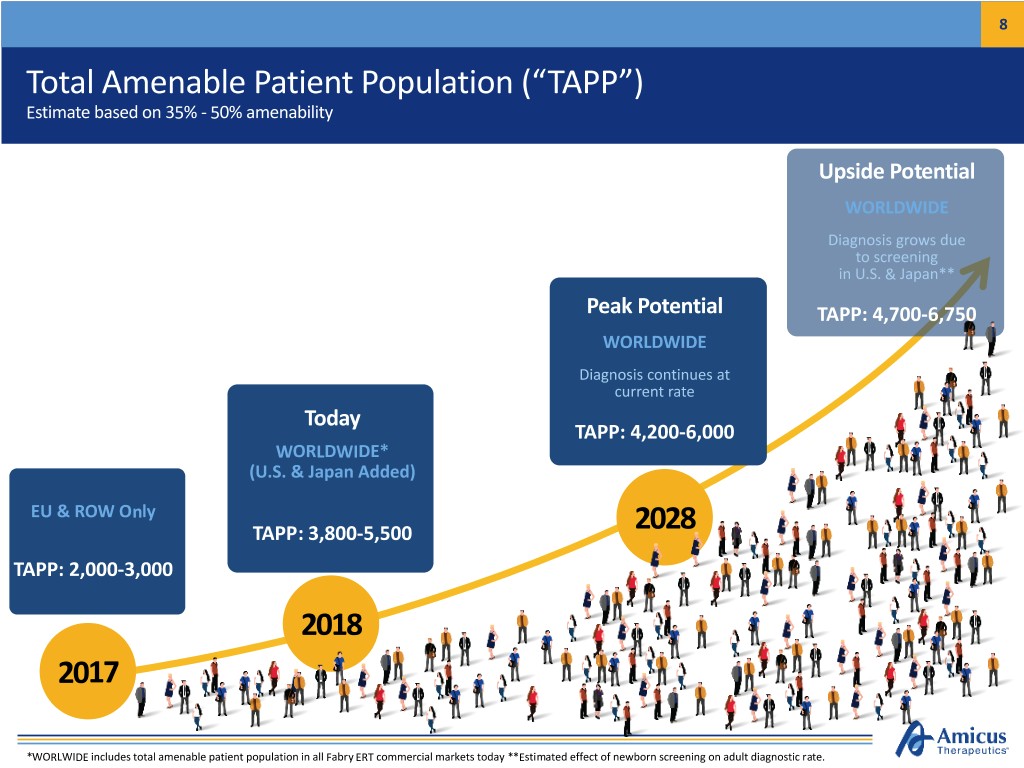
8 Total Amenable Patient Population (“TAPP”) Estimate based on 35% - 50% amenability Upside Potential WORLDWIDE Diagnosis grows due to screening in U.S. & Japan** Peak Potential TAPP: 4,700-6,750 WORLDWIDE Diagnosis continues at current rate Today TAPP: 4,200-6,000 WORLDWIDE* (U.S. & Japan Added) EU & ROW Only TAPP: 3,800-5,500 2028 TAPP: 2,000-3,000 2018 2017 *WORLWIDE includes total amenable patient population in all Fabry ERT commercial markets today **Estimated effect of newborn screening on adult diagnostic rate.

Galafold: Precision Medicine for Fabry Disease 9 Pricing Philosophy Our medicines must be fairly priced and broadly accessible” - Amicus Founding Belief Galafold Pricing . Galafold priced at parity or below to ERT* (without infusion associated costs) . Amicus will limit Galafold price increases to CPI (consumer price index) . Amicus pledges to reinvest a portion of our profits into R&D of new treatments for Fabry disease until there’s a cure *Average ERT price in the United States based on an average adult patient

10 2018 Key Strategic Priorities As of August 2018 Focused on FIVE Key Strategic Priorities in 2018 1 Double Galafold (migalastat) revenue to $80-$90M 2 Secure approvals for migalastat in Japan and the U.S. Achieve clinical, manufacturing and regulatory milestones to advance 3 AT-GAA toward global regulatory submissions and approvals Develop and expand preclinical pipeline to ensure at 4 least one new clinical program in 2019 5 Maintain financial strength

Conclusion 11 Amicus Vision: Delivering for Patients and Shareholders To build a top-tier, fully integrated, global biotechnology company whose medicines treat 5,000+ patients with $1B+ in worldwide sales revenue by 2023 >350 Patients* | $36.9M Global Sales 5,000 Patients* | $1B Global Sales YE17 2023 *Clinical & commercial, all figures approximate

Thank You “Our passion for making a difference unites us” -Amicus Belief Statement PP-GA-ALL-0005-0818
